International leading supplier of wear-resistant materials
中文
0563-4431079

Basic knowledge of crystal structure
1. Crystalline and amorphous
Solid substances in nature are divided into crystalline and amorphous according to the characteristics of particle (atom or molecule) arrangement. A substance whose particles are periodically arranged in three-dimensional space according to a certain law is called a crystal; When the particles are scattered, they are called amorphous. In nature, except for a few substances (such as paraffin, asphalt, ordinary glass, rosin, etc.), the vast majority of electrodeless non-metallic substances are crystals, and metals and alloys are generally crystals.
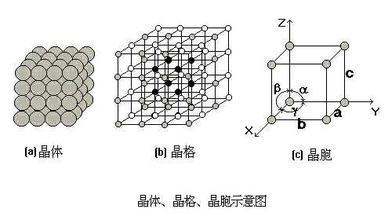
2. Lattice and cell
In order to express the arrangement law of atoms in the crystal, we regard atoms as rigid spheres, and the crystal is formed by stacking these rigid spheres. In order to clearly express the arrangement law of atoms in the crystal, the rigid atoms are simplified into a node, and these nodes are connected with imaginary lines to form a regular space lattice, This kind of spatial geometric lattice used to describe the arrangement law of atoms in the crystal is called lattice, as shown in the following figure. Because the arrangement of atoms in these lattices is periodic, for simplicity, a minimum geometric unit that can reflect the characteristics of the lattice is usually selected from the lattice to analyze the arrangement law of atoms in the crystal. This minimum geometric unit is the crystal cell. The size and shape of crystal cells are often expressed in terms of lattice constants a, B, C and the angle between edges α,β,δ express


0563-4430018





