International leading supplier of wear-resistant materials
中文
0563-4431079

1. Crystal structure of common metals
Among the more than 80 known metals, except a few metals with complex crystal structure, most metals have relatively simple crystal structure, including body centered cubic lattice, face centered cubic lattice and close packed hexagonal lattice.
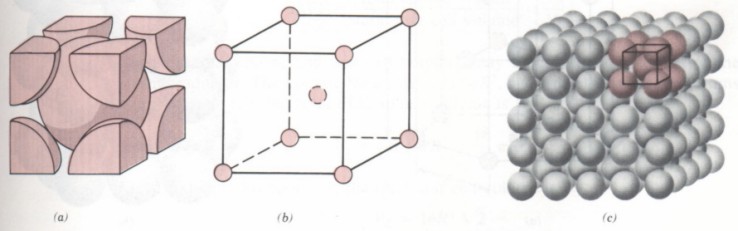
(1) The unit cell of BCC lattice is a cube. Atoms are distributed at the eight top corners of the cube and the center of the cube, as shown in the figure. Lattice constant a = b = C. The included angle of edges is 90 °. Metals with body centered cubic lattice, such as chromium, tungsten, molybdenum, etc.
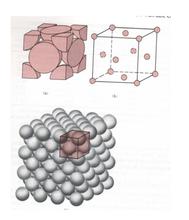
(2) The unit cell of face centered cubic lattice is a cube, and atoms are distributed in the center of 8 vertex angles and 6 faces of the cube. Copper, aluminum, silver, gold and nickel are all such lattices.
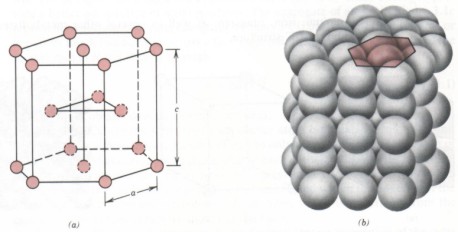
(3) The crystal cell of the closely arranged cubic lattice is a hexagonal cylinder with regular hexagon on the upper and lower ground. There are atoms distributed in the 12 top angles and the centers of the upper and lower bottom surfaces of the hexagonal cylinder, and there are three atoms in the middle of the hexagonal cylinder. There are two lattice constants of closely packed cubic, namely, the side length of regular hexagon and the height of hexacylinder C, and the axial ratio C / a = 1.663. The metals with closely packed hexagonal lattice include magnesium, zinc, etc.


0563-4430018





